Evusheld is not a super vaccine against Covid-19
According to the Ministry of Health, the drug Evusheld is not allowed to be used to prevent Covid-19 for subjects who can receive the Covid-19 vaccine.
On the afternoon of March 18, the Ministry of Health informed about the drug Evusheld – a monoclonal antibody used for prevention Covid-19.
Accordingly, on March 2, 2022, the Ministry of Health issued a license to import Evusheld medicine to meet the special treatment needs of medical examination and treatment establishments.
Up to now, Evusheld has been licensed for circulation in a state of emergency in a number of countries such as the US, France, the United Arab Emirates, Bahrain…
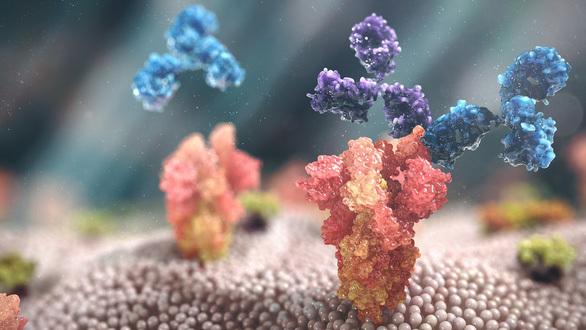
An illustration of the working mechanism of Evusheld – Source: AstraZeneca
The Ministry of Health confirmed that Evusheld is a drug, not a vaccine. Evusheld is not indicated as a substitute for the Covid-19 vaccine in vaccineable cases.
Drug use needs to be adequately evaluated by a physician and closely screened before it is determined to be an appropriate user.
One dose of Evusheld is indicated for the prevention of Covid-19 disease for at least 6 months in adults and children 12 years of age and older, weighing 40 kg or more. These subjects are not currently infected with SARS-CoV-2, have not had contact with a confirmed SARS-CoV-2 infected person. At the same time, it must be in one of the following cases:
– Having moderate to severe immunodeficiency due to disease or using immunosuppressive drugs and treatment regimens, failing to create a satisfactory immune response to the Covid-19 vaccine.
Or, can’t get any of the available Covid-19 vaccines because of a history of serious reactions to any component of the Covid-19 vaccine.
Currently, Evusheld has not been approved for use in subjects being treated for Covid-19, or for post-exposure prophylaxis of Covid-19 in people who have been in contact with an infected person. SARS-CoV-2.
In Vietnam, Evusheld is granted an import license for use in medical examination and treatment establishments. The patient must be informed by the medical facility about the status of the drug’s license application and the facility can only use the drug with the consent of the patient or family member.
“Thus, Evusheld is a drug, not a ‘super-vaccine’, so it is not allowed to use Evusheld to prevent Covid-19 for those who can be vaccinated,” the Ministry of Health affirmed.
The Ministry of Health confirmed that Evusheld is a drug, not a super vaccine.
Previously, AstraZeneca’s Evusheld was the world’s first monoclonal antibody licensed for production for prophylaxis against Covid-19 and was approved to be imported into Vietnam by the Ministry of Health.
The drug can prevent the disease from becoming severe in high-risk groups such as immunocompromised people, who are unable to produce antibodies despite receiving a full dose of the Covid-19 vaccine, or those who cannot receive the Covid-19 vaccine. For example, patients with immunodeficiency, cancer, autoimmune arthritis, organ transplant or systemic diseases…
According to VietNamNet
at Blogtuan.info – Source: 2sao.vn – Read the original article here


