How does the Covid-19 Nanocovax vaccine that Mr. Ho Nhan devotes to?
Mr. Ho Nhan, General Director of Nanogen Company, is considered the father of Vietnam’s first “domestic” Covid-19 vaccine. However, up to now, this vaccine is still waiting for marketing authorization.
Mr. Ho Nhan is an American overseas Vietnamese, born in 1966. In 1997, Mr. Nhan established and assumed the position of General Director of Nanogen Company. In 2008, he returned to Vietnam to settle down.
Nanogen Pharmaceutical Biotechnology Joint Stock Company is headquartered and has a factory in Thu Duc City Hi-Tech Park, Ho Chi Minh City.
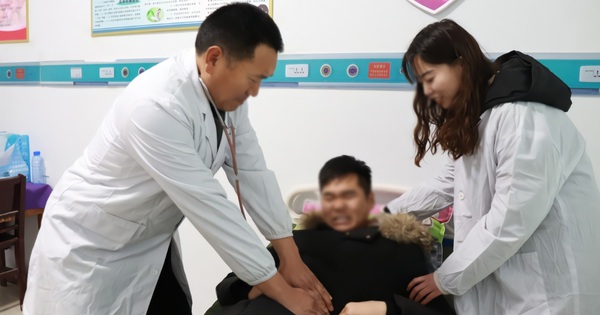
 |
| Mr. Ho Nhan, Chairman and CEO of Nanogen. Photo: Forbes |
Nanogen’s main field of activity is the production of bio-pharmaceutical materials and finished products of specific therapeutic injections from recombinant DNA/Protein technology. The company has 4 factories, 1 research and development center, with a capacity of more than 50 million products/year and distributes products to 15 different countries.
Mr. Ho Nhan shared, Nanogen strives to research and develop high-quality drugs at reasonable costs for patients in Vietnam and around the world. Nanogen is committed to applying technological achievements to create special medicines for consumers.
Mr. Nhan has been interested since Nanogen published the research Covid-19 vaccine The first “noi” for Vietnamese people. Nanocovax vaccine of Nanogen Company was organized to conduct research right when the Covid-19 pandemic began to break out strongly in the world.
In March 2020, when the Government assigned the Ministry of Science and Technology to find and assign businesses with enough potential to research and prepare a vaccine against Covid-19, the company officially started.
On May 15, 2020, the Minister of Science and Technology signed Decision No. 1256/QD-BKHCN to officially order Nanogen Company to make a vaccine against Covid-19.
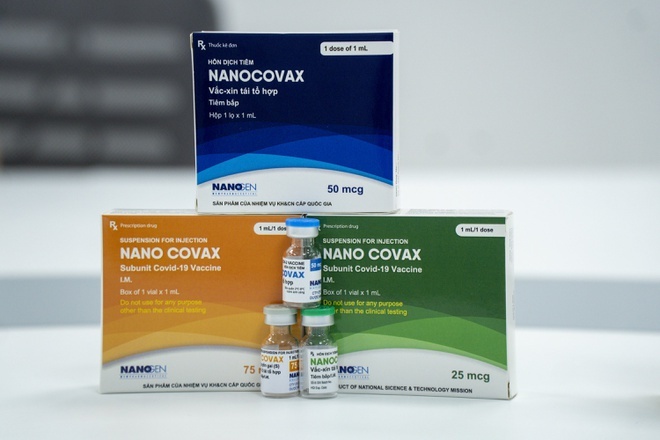 |
| Nanocovax vaccine was expected to be the first “domestic” Covid-19 vaccine to be licensed for emergency circulation. |
Nanocovax vaccine is manufactured based on recombinant protein technology. The most important and time-consuming thing was Nanogen’s selection of a gene fragment, a protein fragment to design a gene segment suitable for the Covid-19 variants that were spreading rapidly in Vietnam at that time. Since then, a vaccine against Covid-19 has been produced
On December 9, 2020, the Biomedical Ethics Council of the Ministry of Health held a final meeting to review and approve the human trial of Covid-19 vaccine in Vietnam by Nanogen. On December 10, 2020, Nanogen coordinated with the Military Medical Academy to recruit volunteers to participate in phase I of human trials.
This vaccine started testing phase 1 from December 18, 2020, phase 2 from February 26, 2021 and phase 3 officially from June 11, 2021.
Phase 1, experimental injection for 60 people at Military Medical Academy, Hanoi. Phase 2, human clinical trial in Hanoi and Long An. Phase 3, conducting trials on 13,000 volunteers in many provinces.
The North is led by the Military Medical Academy, the South is deployed by the Pasteur Institute in Ho Chi Minh City.
Notably, in June 2021, Nanogen sent an official dispatch to the Prime Minister, applying for an urgent conditional license for the Nanocovax vaccine. Nanogen said, the selling price of the vaccine is only about 120,000 VND per dose and does not change, not for profit.
Recently, the Ministry of Health said that Vietnam currently has 3 vaccine candidates for Covid-19 that are domestically produced and technology transferred and are conducting clinical trials in phase 2 and phase 3. In which, Nanocovax vaccine is being evaluated for registration documents for circulation.
Currently, this vaccine is continuing to collect data to evaluate the safety, immunogenicity and protective efficacy of the phase 2 and phase 3 clinical trials of the vaccine according to the research protocol.
The Advisory Council for the issuance of circulation registration certificates of drugs and medicinal ingredients met to evaluate the application for circulation registration of this vaccine and requested the manufacturing company and research units to confirm the accuracy of the data. research material.
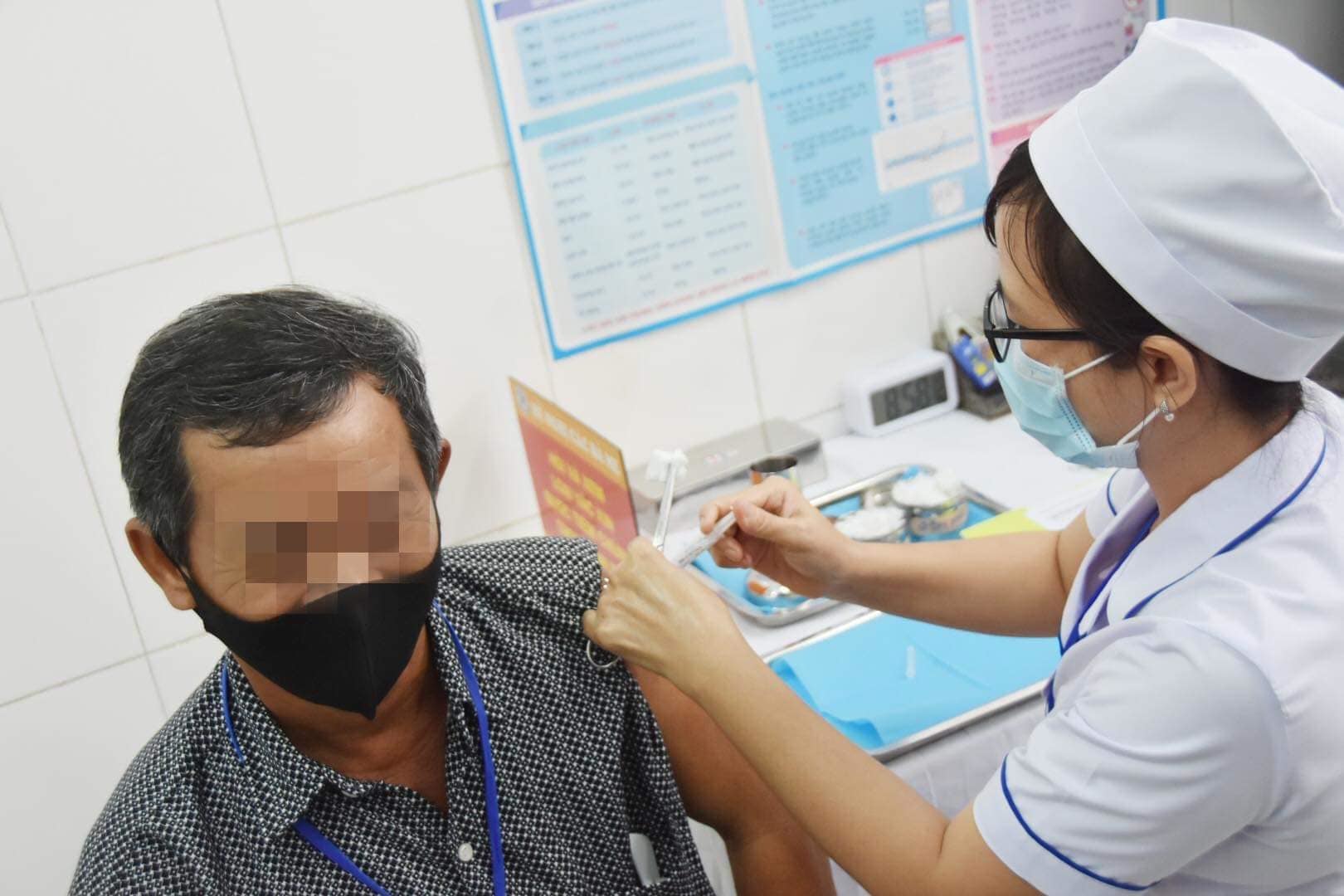 |
| Volunteers participate in the phase 2 human trial of the Covid-19 Nanocovax vaccine in Long An. |
On March 18, Deputy Prime Minister Vu Duc Dam asked the Ministry of Health to urgently implement the Prime Minister’s direction according to Document No. 1027/VPCP-KGVX dated February 17, 2022 of the Government Office. government and other relevant documents; continue to guide and support enterprises in all cases as prescribed by law.
Thus, up to now, when the pandemic in Vietnam and the world has made many new developments, the Nanocovax vaccine is still waiting for the authorities to review and evaluate the application for circulation registration.
Fuji (synthetic)
at Blogtuan.info – Source: vietnamnet.vn – Read the original article here