Open portal to register for injection of monoclonal antibodies against COVID-19 Evusheld
Monoclonal antibodies Eveld. Photo: BVCC
The system of Tam Anh General Hospital in Hanoi and Tam Anh General Hospital in Ho Chi Minh City. Ho Chi Minh City started receiving information about injection registration from today, March 22, 2022.
Assoc.Prof.Dr.BS Tran Quang Binh – Professional Director of Tam Anh General Hospital in Ho Chi Minh City. Ho Chi Minh shared: Evusheld is the world’s first monoclonal antibody against COVID-19 that has been approved by FDA (USA) for emergency use, and has been approved by many countries around the world for timely protection. vulnerable people to COVID-19, especially when the Omicron variant is widespread. The monoclonal antibody Evusheld has been licensed for circulation in a state of emergency in a number of countries such as the US, France, the United Arab Emirates, Bahrain…
The World Health Organization (WHO), estimates that around 2% of the global population is vulnerable to COVID-19 due to immunodeficiency moderate and severe cases such as HIV, cancer, organ transplants (heart transplant, liver transplant, kidney transplant), long-term and high-dose corticosteroid use… In Vietnam, according to the Ministry of Health’s statistics, the country currently has nearly 230,000 people infected with HIV; about 180,000 new cases and 120,000 cancer deaths every year…
Therefore, the monoclonal antibody Evusheld was licensed to Vietnam at this time has a profound human meaning. Because the vulnerable group is in need of an extra shield to protect their health against the spreading SARS-CoV-2 variants while it is difficult to have a good immune response, even though they have been vaccinated with a full dose of vaccine, even both booster dose. Many countries around the world are expecting that Evusheld will help disadvantaged groups to overcome the COVID-19 storm.
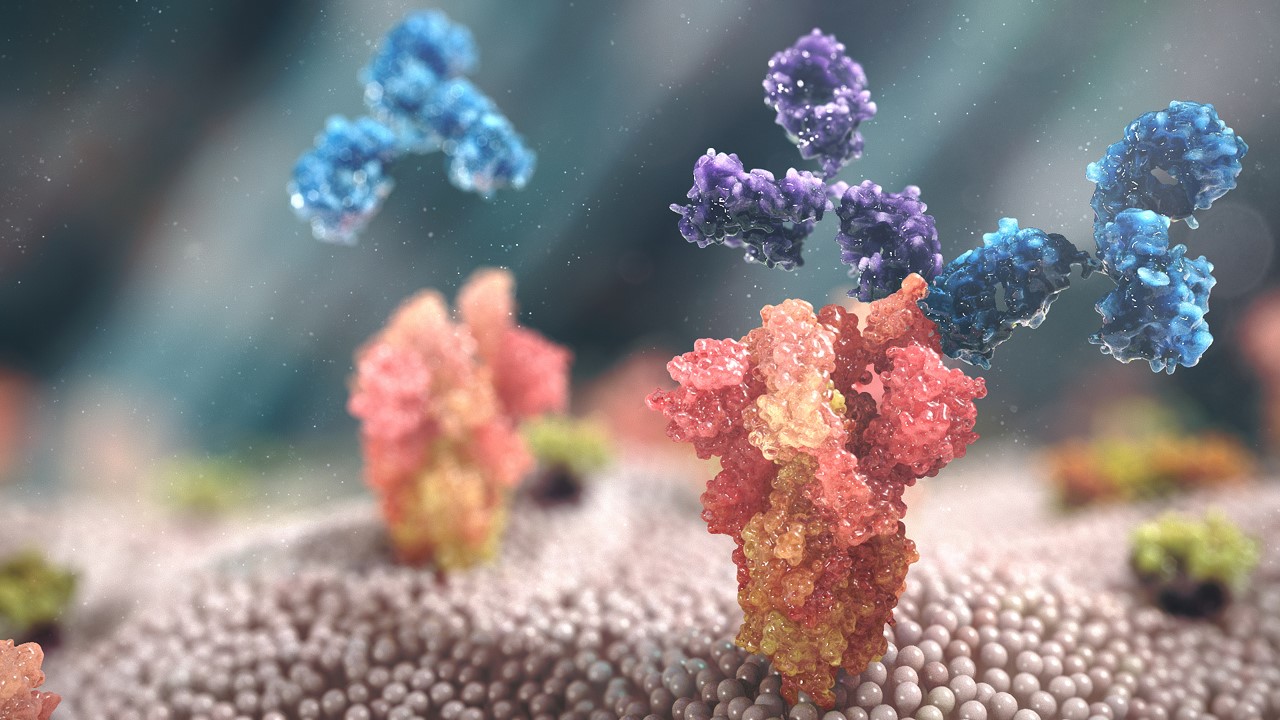
The image illustrates the mechanism of operation of the Evosheld. Source AstraZeneca
However, it should be further noted: The monoclonal antibody Evusheld does not replace the COVID-19 vaccine for the community, but is only aimed at people who are immunocompromised or are unable to respond adequately, or are not immune. produce adequate antibodies after a full dose of vaccine has been administered and to people who are unable to receive the vaccine because of a serious adverse reaction to the COVID-19 vaccine.
Assoc. Prof. TS.BS Tran Quang Binh also said that Evusheld monoclonal antibodies need to meet criteria such as being 12 years old or older, weighing 40kg and not being infected with SARS-CoV-2 at the time of injection. and have not had contact with a person infected with SARS-CoV-2; and must be in one of the following cases:
– Have moderate to severe immunodeficiency due to a medical condition, or use of immunosuppressive drugs or regimens and are unlikely to induce an adequate immune response to the COVID-19 vaccine -19.
– Any currently available COVID-19 vaccine cannot be given because of a history of serious adverse events (eg, severe allergy) with any component of the COVID-19 vaccine.

Nursing at Tam Anh General Hospital in Ho Chi Minh City. Ho Chi Minh City measures blood pressure for customers at the initial assessment table for the safest examination and vaccination. Photo: BVCC
Evusheld is a monoclonal antibody researched and manufactured by pharmaceutical company AstraZeneca (United Kingdom – Sweden), which has provided hundreds of millions of doses of COVID-19 vaccine globally. From more than 1,500 antibodies of people recovering from COVID-19 Around the world, scientists have selected the two most potent antibodies to create Evosheld. This is the world’s first COVID-19 prophylactic monoclonal antibody approved by the FDA.
The monoclonal antibody Evusheld is licensed by the Ministry of Health for Tam Anh General Hospital System to import and use through the distribution of Vietnam Vaccine Company (VNVC) and AstraZeneca Company.
at Blogtuan.info – Source: Afamily.vn – Read the original article here

![Opus - life is life [Live 2002] 2 Opus – life is life [Live 2002]](https://i.ytimg.com/vi/2Jb31-hjGCk/maxresdefault.jpg)

