Detecting suspected fake drugs for cancer treatment
(Dan Tri) – The Ministry of Health has just announced the discovery of some drug samples on the label of Stivarga and Xarelto, suspecting fake drugs being sold on the market and some websites.
Previously, the Drug Administration of Vietnam (Ministry of Health) received information from T&G Law Firm (authorized by Bayer Intellecture Property GmbH) reporting the detection of suspected counterfeit drug samples, on label reads:
Product name: Stivarga 40mg (film kapli tablet, Regorafenib) Bayer Türk Kimya San. Ltd. Şti Parti No.: BXJL3D1 Son Kull. Ta.: 04.2024.
Product name: Xarelto 10mg (film kapli tablet, Rivaroksaban) Bayer Türk Kimya San. Ltd. Şti; Parti No.: 9LB04017 Son Kull. Ta.: 04/2022.
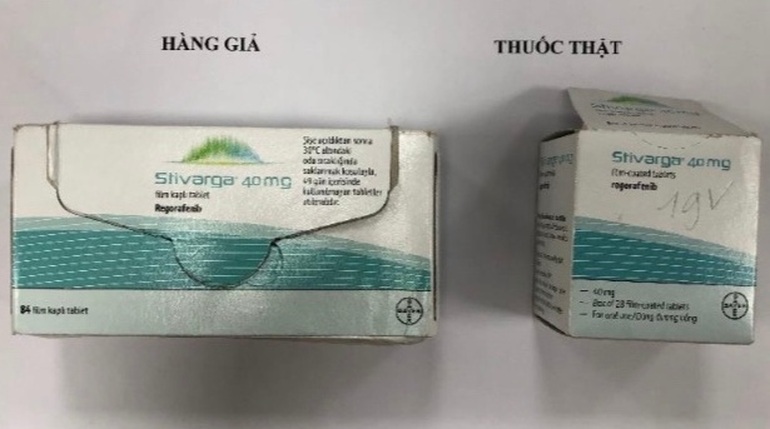
Product name: Xarelto 15mg (film kapli tablet, Rivaroksaban) Bayer Türk Kimya San. Ltd. Şti; Parti No.: BLB02500 Son Kull. Ta.: 03/2024.
Product name: Xarelto 20mg (film kapli tablet, Rivaroksaban) Bayer Türk Kimya San. Ltd. Şti; Parti No.: ALB08020 Son Kull. Ta.: 11/2023.
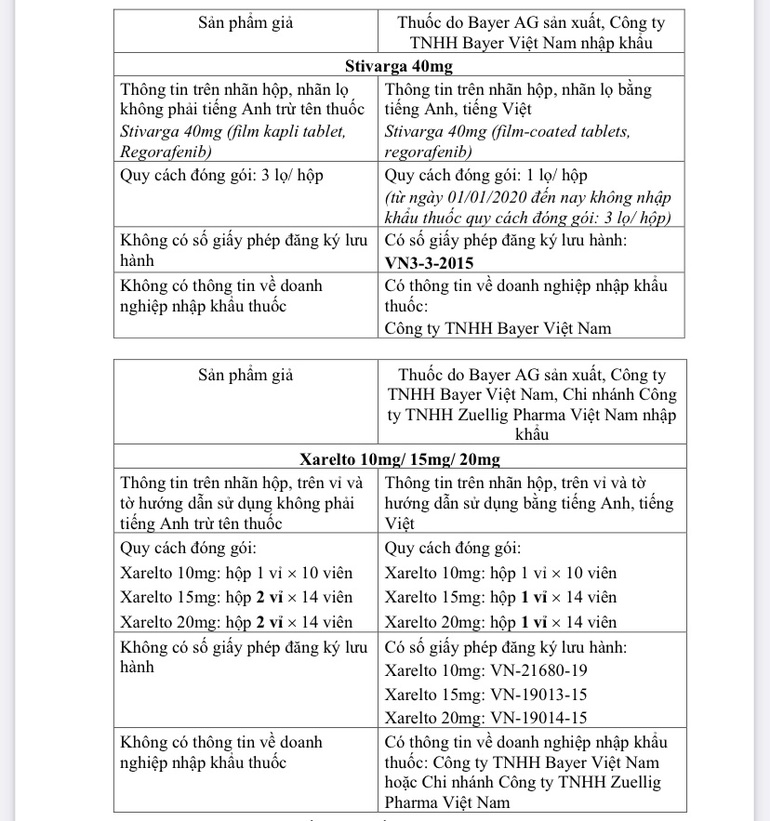
Samples of suspected counterfeit drugs were found on the market and some websites (https://healthyungthu.com, https://nhathuoclp.com, https://thuocdactrigan.com). According to the Company’s report and compared with the drug samples provided by T&G Law Firm, the above-mentioned products have different signs compared with the drug samples manufactured by Bayer AG, Bayer Vietnam Co., Ltd. or Branch of Zuellig Pharma Vietnam Co., Ltd. imports and distributes.
Therefore, to ensure the safety of users, the Drug Administration of Vietnam recommends that the Departments of Health of the provinces, cities, health departments and Bayer AG inform business and drug use establishments about the specific characteristics. Points, signs to distinguish between suspected counterfeit products and drugs manufactured by Bayer AG, imported by Bayer Vietnam Co., Ltd or Zuellig Pharma Vietnam Co., Ltd.’s branch.
At the same time, coordinate information to drug dealers and users and the people to know so as not to trade and use counterfeit products.
The Drug Administration also requires the manufacturer of Bayer AG or the drug registration facility Stivarga 40mg, Xarelto 10mg, Xarelto 15mg, Xarelto 20mg to provide relevant documents and documents and promptly report directly to the Department.
Among these, Stivarga has the active ingredient regorafenib, which helps treat cancer rectal cancer and liver cancer, widely used in hospitals. Xarelto is indicated for the treatment and prevention of thrombosis.
at Blogtuan.info – Source: dantri.com.vn – Read the original article here