Covid-19 epidemic situation on March 30, 2022: The US licensed the injection of 4 vaccines of Pfizer and Moderna
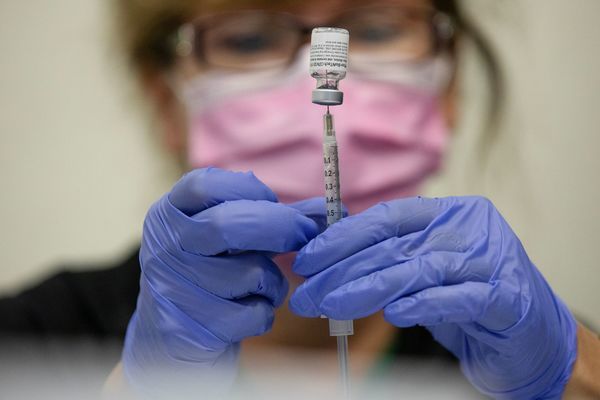
The US Food and Drug Administration (FDA) on March 29 approved the use of Pfizer/BioNTech and Moderna’s Covid-19 vaccines for the fourth dose for people over 50 years old.
According to the Reuters news agency, the US FDA said that the fourth round of injections for most people who have received previous doses of Pfizer / BioNTech and Moderna vaccines will be administered within at least 4 months after vaccination. most recent dose. This is to provide better protection against the risk of severe Covid-19 infection and hospitalization.
The agency also allows the 4th dose of Covid-19 vaccine to be given to younger people with compromised immune systems, namely over 12 years old for Pfizer/BioNTech vaccines and over 18 years old for Pfizer/BioNTech vaccines. Moderna’s vaccine.
 |
| Photo: Reuters |
The move comes after scientists were concerned about the spread of the BA.2 subline of the Omicron variant, which has been the cause of an increase in the number of new Covid-19 infections in many countries. In the US alone, BA.2 currently accounts for 55% of infections with detected corona virus variants.
Data from the US Centers for Disease Control and Prevention (CDC) shows that the number of new Covid-19 infections in the country has decreased sharply compared to January, but tended to increase slightly in the past week. Pfizer CEO Albert Bourla said that the FDA’s emergency use authorization of the company’s vaccine for the fourth shot will help address many of today’s needs. The company is also focusing on developing an updated version of the Covid-19 vaccine, which is not only capable of preventing current variants of the corona virus, but also has long-term effectiveness.
Shanghai tightens blockade
On March 29, Shanghai, China’s most populous city, tightened the first of two phases of blockade to prevent the Covid-19 pandemic. Accordingly, people in some residential areas are required to stay at home to be tested for Covid-19, in the context that the number of daily infections in the city has exceeded 4,400 cases.
According to Xinhua News Agency, this city of about 25 million people has entered the second day of implementing a comprehensive blockade order. The first phase of the blockade in Shanghai, which lasted from 5 a.m. on March 28 to 5 a.m. on April 1, was applied to areas east and south of the Huangpu River, including Pudong and adjoining regions. Phase 2, from 3:00 a.m. on April 1 to 3:00 a.m. on April 5, will be deployed in inner-city districts west of the Huangpu River.
Shanghai officials affirmed that the above measures are to limit the spread of corona virus, protect people’s health and life, and achieve the goal of bringing the number of community infections to zero soon.
Chinese officials apologize for leaving people without food in the middle of the blockade
Liu Renyuan, Deputy Party Secretary of Changchun City, Jilin Province (China), on March 29 said that the city’s 2 wholesale food markets were closed due to the blockade order to prevent Covid-19, leading to shortage of food for the people.
“We are particularly worried and outraged about this matter, and would like to deeply apologize to the public for the inconvenience and inconvenience it has caused,” Liu said at a press conference.
The official also said that in order to reduce the shortage of food sorting and delivery staff, the Jilin provincial government has prepared 1,000 tons of vegetables packed in bags to send to Changchun every day. City leaders said they will ensure to prevent the increase in vegetable prices.
Changchun City and other parts of Jilin province are battling a severe Covid-19 outbreak, with thousands of new infections every day since mid-March.
The entire Jilin province has been placed under lockdown since March 14 and conducting mass testing to find ways to prevent this serious outbreak. Truong Xuan alone has conducted more than 10 tests with all 8.5 million residents of the city.
EU will evaluate Hipra’s Covid-19 vaccine
The European Medicines Agency (EMA) announced on March 29 that it had begun a real-time evaluation of the Covid-19 vaccine candidate from Spanish pharmaceutical company Hipra.
This decision was based on preliminary results from clinical studies that compared the Hipra vaccine’s coronavirus immune response with the Pfizer/BioNTech vaccine. However, the EMA did not say when the review will be completed.
Hipra is developing a protein-based Covid-19 vaccine that will serve as a booster dose for adults who have already received a full dose of another Covid-19 vaccine.
The decision to start the real-time review comes almost two months after the Spanish pharmaceutical agency authorized Hipra to carry out late-stage trials of its Covid-19 vaccine.
The Spanish pharmaceutical company is expected to be able to produce nearly 600 million doses of the Covid-19 vaccine this year, and double that number by 2023.
>>> Update Covid-19 epidemic situation Latest
Vietnamese-English
at Blogtuan.info – Source: vietnamnet.vn – Read the original article here


