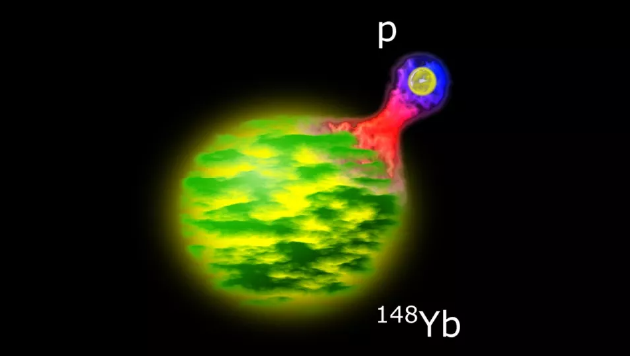
decomposes in 450 nanoseconds
Writing in the March 16 issue of Physical Review Letters, the researchers report they have created an exotic nucleus that can lose half its radioactivity (decay into other elements) in as little as 450 nanometers. second.
Lutetium (Lu) is a rare earth element, a silvery metal in its natural form, with atomic number 71. Usually, lutetium occurs in the Earth’s crust along with the metallic element ytterbium (Yb). Lutetium-176 is a relatively common radioactive isotope (2.5% of all lutetium isotopes) with a half-life of about 38 billion years and can be used to measure the age of meteorites. Lutetium on Earth consists of two isotopes, lutetium-175 and lutetium-176, of which only lutetium-175 is stable.

Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery-white metal that corrodes in dry air, but corrodes in moist air. Lutetium is the last element in the lanthanide series, and has traditionally been placed in the place of the rare earths. Lutetium is generally considered to be the first element of the 6th period transition metals by researchers, however there is still some controversy on this point.
In the 1980s, scientists observed the decay of the lutetium isotope, lutetium-151, and discovered that it released a proton from the nucleus in its ground state. The ground state is the state in which the atom is at its lowest energy, when electrons move in orbits closest to the nucleus, which is also the most stable configuration of the atom. Proton emission is very rare, and lutetium-151 is the first isotope observed to emit protons in a stable decaying ground state.

The name Lutetium, which is derived from the Latin Lutetia (Paris), was independently discovered in 1907 by the French scientist Georges Urbain, the Austrian mineralogist Baron Carl Auer von Welsbach and the American chemist Charles James . Lutetium-176 is a relatively abundant (2.5%) radioactive isotope with a half-life of about 38 billion years, used to determine the age of minerals and meteorites. Lutetium often occurs with the element yttrium and is sometimes used in metal alloys and as a catalyst in various chemical reactions.
Studying proton decay allows researchers to peer inside atomic nuclei to understand how protons and neutrons bind together. As part of this study, Kale Olanin, a postdoctoral researcher in physics at the University of Jyväskylä, and his colleagues created a new isotope of lutetium, lutetium-149, which has 71 protons in it. nucleus and 78 neutrons.

Scientists initially found the element in an impure form in the mineral ytterbia, which the Swedish chemist Jean Charles Galissard de Marignac (and most other scientists) assumed was completely contains the element ytterbium. Marignac’s separation of lutetium from ytterbium was first described by Urbain and given his name. He chose the names neoytterbium (new ytterbium) and lutecium for the new element, but the name neoytterbium was eventually changed back to the element ytterbium and in 1949 the 71st element was changed to lutetium. The first-person controversy was written in two papers in which Urbain and von Welsbach accused each other of published results being influenced by each other’s published research. Accordingly, The Commission on Atomic Mass, the organization responsible for naming new elements, settled the dispute in 1909 accepting the first discovery of Urbain and the names of the new elements. his official name. The issue regarding this decision is that Urbain is one of the four members of this Council.
They found that lutetium-149 is more exotic than lutetium-151. First, the nucleus of lutetium-149 is not a neat sphere, but a squashed ellipsoid that looks a bit like a pumpkin. This phenomenon is known as “mass distortion”, and lutetium-149 is the most distorted nucleus ever measured.
One of the radioactive isotopes of lutetium (Lu-176) is commonly used in nuclear technology to date meteorites. Lutetium is always symbiotic with the element ytrium and is sometimes used in alloys and as catalysts in various chemical reactions. Because of its rarity and high price, lutetium has few commercial applications. However, non-radioactive lutetium can be used as a catalyst in petroleum refining and can also be used in alkylation, hydrogenation, and polymerization.
The half-life of lutetium-149 is also significantly shorter than that of lutetium-151’s 80.6 milliseconds. The researchers say they created this new isotope of lutetium by burning nickel-58, an isotope of nickel, and ruthenium-96, an isotope of ruthenium. In the study, lutetium-149 decayed to ytterbium-148, which itself is not long-lived, with a half-life of 250 milliseconds. It is possible in the future to make lutetium-148, the researchers say, which may have a longer lifespan than lutetium-149.
at Blogtuan.info – Source: genk.vn – Read the original article here
